Types of medical software

The FDA has defined stand-alone medical software in the Preamble Section AA Special Requirements for Stand-Alone Software—Final § 801.50 of the Unique Device Identification (UDI) System Regulations as ‘medical software that is itself a medical device and is not a component, part, or accessory of a medical device’.
The FDA has also published additional guidance documents about specific types of software that are used in health care that might be regulated. For example, ‘Medical Device Data Systems’ (MDDS) are defined as:
… hardware or software products intended to transfer, store, convert formats, and display medical device data. A MDDS does not modify the data or modify the display of the data, and it does not by itself control the functions or parameters of any other medical device. MDDS may or may not be intended for active patient monitoring.
These applications might or might not be regulated, depending on other functions of the application.
In general, if the software is solely intended to transfer, store, convert and display, then it is not subject to regulation. Examples include software that stores patient data for review at a later time, software that converts data into a format that can be printed and software that displays a previously stored electrocardiogram for a patient.
Note that the software does not modify the data and the software does not control the functions or parameters of a device. If the software has those features, it is likely to be a device. For example, software intended to generate alarms or prioritize patient-related information on a display would likely be considered a medical device. Similarly, software that detects and highlight abnormalities (computer-assisted detection (CADe)) or software that assesses associated disease severity (computer-assisted diagnosis (CADx)) is considered a device by the FDA and is subject to regulatory focus.
Similarly, ‘clinical decision support’ (CDS) software is defined as follows:
Clinical decision support (CDS) provides clinicians, staff, patients or other individuals with knowledge and person-specific information, intelligently filtered or presented at appropriate times, to enhance health and health care. CDS encompasses a variety of tools to enhance decision-making in the clinical workflow. These tools include computerized alerts and reminders to care providers and patients; clinical guidelines; condition-specific order sets; focused patient data reports and summaries; documentation templates; diagnostic support, and contextually relevant reference information, among other tools.
Because of the variety of CDS applications, as well as an evolving regulatory landscape, some CDS software might be regulated by the FDA, other CDS software might be regulated by the FDA but under ‘enforcement discretion’, and some CDS software might not be regulated as medical devices. Although the FDA released draft guidance on this topic in September 2019, final guidance has not been published.
Factors that affect whether or not the CDS software is regulated include:
• Is the intended user a health care provider (HCP)?
• Can the user independently review the basis of the CDS software information?
• What is the state of the health care situation or condition (is the patient state non-serious, serious or critical)?
Table 1 from the draft CDS guidance shows how these factors affect the device regulation:
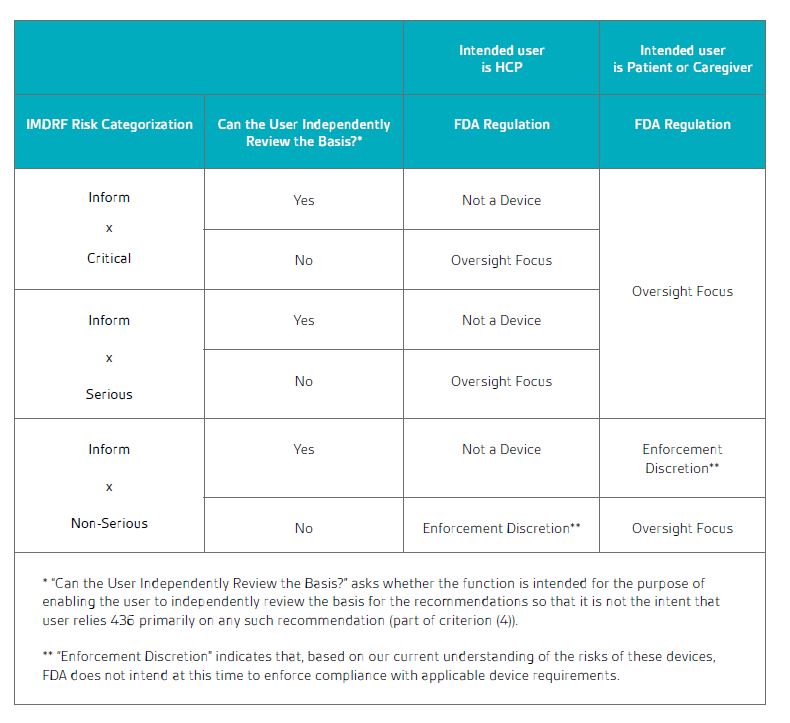
Table 1 Summary of regulatory policy for CDS software functions
Source: Table 3 from Clinical Decision Support Software Draft Guidance for Industry and U.S. Food & Drug Administration Staff,
September 2019
The draft FDA guidance provides a large number of examples of the application of this table.
Finally, there are a number of software applications that run on people’s phones or tablets. The FDA published mobile medical apps (MMA) guidance in 2013, which was updated in 2015 and again in 2019.7 Like ther products, whether or not a mobile application is a regulated device depends on the product’s intended use.
If the software is intended to perform a medical device function, then it is a medical device, regardless of the platform.
This is an excerpt from the forthcoming BSI medical devices white paper, Software as a medical device: A comparison of the EU’s approach with the US’s approach. To browse our collection of medical device white papers, please visit the Insight page on the Compliance Navigator website.
The Compliance Navigator blog is issued for information only. It does not constitute an official or agreed position of BSI Standards Ltd or of the BSI Notified Body. The views expressed are entirely those of the authors.

