Performance evaluation plan

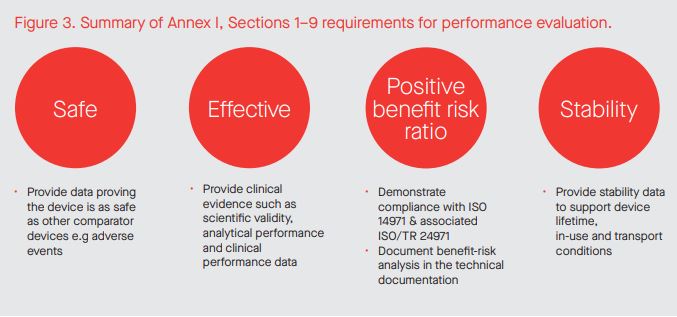
Although the concept and requirements of performance evaluation have not really changed over 20 years, the required technical documentation has changed radically both in terms of content and amount required in the IVDR compared to the IVDD. The contents of two new IVDR documents are explicitly described in Annex XIII: the performance evaluation plan (PEP) and the performance evaluation report (PER). The PEP provides the manufacturer the opportunity to take the design inputs for performance and safety together with risk management outputs and align them to the IVDR requirements described in Annex I, Sections 1–9. Sections 1–9 encompass safety, risk, performance (i.e. scientific validity, analytical and clinical performance) and stability (lifetime, transport/ storage and in-use) as illustrated in Figure 3.
By aligning design inputs with IVDR requirements, the manufacturer can then plan how to generate the appropriate data required to support each performance characteristic. Although the requirements for performance and stability may seem obvious, clinical evidence data to support safety and risk can be less so. This is where the manufacturer has to think outside their own testing environment and bring in real-world experience of the device (or similar related devices), which can be used as additional clinical evidence to support the safety and benefit-risk ratio of the device.
This is discussed further in the following clinical performance section. The requirements for the PEP include an overview of the design phases within which each data set will be generated, so there is an expectation of an integration of this document with the design and development process.
For legacy devices (those already on the market under IVDD), this may present a challenge for some manufacturers. A PEP is still required, but the approach to gathering clinical evidence will differ from that for new devices. It is expected that manufacturers re-appraise the clinical evidence they hold for a device to determine its suitability under IVDR. This may mean that previous clinical studies performed for the device do not meet Annex XIII/BS ISO 20916:2019 requirements (see Clinical performance below), or additional data has been gathered since placing the device on the market under IVDD. Furthermore, the intended purpose under IVDR may have subtlety changed compared to IVDD, thereby impacting the clinical evidence required. Manufacturers should describe how performance evaluation data will be reviewed in the PEP and determine if there are any gaps to be filled to meet IVDR requirements.
This is an excerpt from the forthcoming white paper Performance evaluation under IVDR. To download our other medical device white papers, please visit the Insight page on the Compliance Navigator website.
The Compliance Navigator blog is issued for information only. It does not constitute an official or agreed position of BSI Standards Ltd or of the BSI Notified Body. The views expressed are entirely those of the authors.

