Nanomaterials and functional coatings
In the new applications of nanoparticles being used for diagnosis or therapy, the ‘functional coating’ plays a key role. There are also many issues with implants in the body, even if they are only temporary, such as with catheters, because of bioincompatibility, infection, mechanical wear and so forth where coatings play a key part. Nanocoating materials can now be designed to mitigate against most of these risks. For example it is possible to design both active and passive antimicrobial coatings for catheters and stents and so forth; it is possible to protect prosthetic joints and moving parts with nanocoatings to reduce friction and wear; and it is possible to design coatings that resorb into soft or hard tissue. At a more pragmatic level, there is growing evidence that nanoparticles can be used to design surface coatings for benches, handles, keyboards and so on, with antimicrobial properties and this could have immediate application in clinics and hospitals.
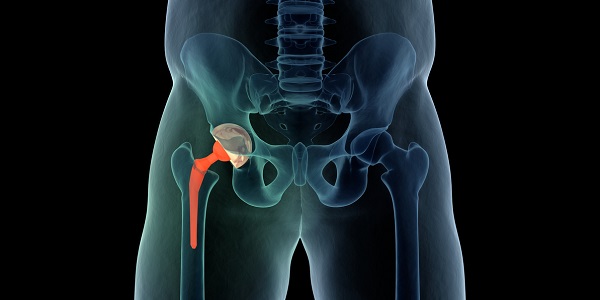
Biocompatibility can be defined as the ‘acceptance of an artificial implant by the surrounding tissues and by the body as a whole’ and this places constraints on the approaches that can be used. There are also going to be differences between the nature of the body tissues, so hard tissue such as bone will have very different requirements to soft tissue environments. First, if we look at the requirements of hard tissues, where metals such as stainless steel and titanium alloys are frequently used, there are issues associated with wear and corrosion and the general interaction with living cells. To overcome these, nano-composite materials are being increasingly deployed and these should have the following characteristics: they should adhere strongly to the metal implant, and inhibit wear and corrosion, and the body-contact surfaces should encourage bone cell growth, with porous hydroxyapatite as the most favoured material. It should be emphasized that there is a tendency for these coatings to consist of several layers, that is: adhesion layer, anticorrosion/strength layer followed by the biocompatible layer and they have to be made under strictly controlled conditions. Soft tissue coatings have very different requirements and characteristics but as in the earlier case there has to be some matching of the mechanical properties and in this case it is elasticity, and the ability to follow deformation. There has to be some element of similarity with the surrounding soft tissues, so collagen and other polymeric biomaterials can be used.
The scientific literature is full of methods to put some molecule recognition entities on the surface of particles that are intended for either imaging diagnostics or drug delivery. These entities range from fragments of DNA/RNA to antibodies and aptamers or smaller molecular units. There is very little quantification about the efficacy of these approaches, apart from some proof, from the imaging perspective that they have attached to the intended target. In other words, we do not know the ‘sticking probabilities’ of these functional nanoparticles. The situation is also complicated by the addition of poly-ethylene glycol (PEG) to help increase the circulation time in the blood. So, the role and behaviour of PEG is another uncertainty in this area.
There is currently growing interest in rendering surfaces in hospitals and clinics and surfaces of medical equipment that come into contact with patients to have some antimicrobial action. There are really two approaches to achieve this aim, one is to prevent adhesion of bacteria to a surface and inhibit biofilm formation and the other is to kill the bacteria by some means. Traditionally this was achieved by employing metals such as zinc, copper or brass, where antimicrobial action is provided by the release of metal ions that kill or inhibit bacteria. This solution is tending to come back into fashion, but there are many plastic fittings and furnishings already in healthcare centres, and there will be new innovations to render such surfaces to be antimicrobial. An untreated plastic surface is often ideal for biofilm formation and antimicrobial attachment. The current approaches that are being tried include the use of silver nanoparticle composites, again, where the metal ions released perform the bacterial killing; very fine nanoparticles (<3 nm) of gold often in conjunction with a dye such as crystal violet; fine particles of the anatase form of titanium dioxide which is a potent generator of free radicals when exposed to light. The latter two methods are attractive because they rely on free radicals or possibly energetic electrons to do the microbial killing. It is unlikely that microbes can evolve to counteract these effects.
This is an excerpt from the white paper "Nanotechnology: What does the future look like for the medical devices industry?". To download our other medical device white papers, please visit the Insight page on the Compliance Navigator website.
Request more information today for a call back from a member of our sales team so that you can get a better understanding of how Compliance Navigator can meet your needs.
The Compliance Navigator blog is issued for information only. It does not constitute an official or agreed position of BSI Standards Ltd or of the BSI Notified Body. The views expressed are entirely those of the authors.

