Person responsible for regulatory compliance (PRRC)- MDR/IVDR Article 15

With the Medical Device Regulation – MDR (EU 2017/745) and In Vitro Diagnostic Regulation – IVDR (EU 2017/746), European regulators aim to ensure companies have a regulatory expert – a Person Responsible for Regulatory Compliance (PRRC) – at their disposal, to ensure that the company is meeting certain specific EU requirements.
The regulators felt it was important to have an identified individual as PRRC to ensure the compliance of released devices, as well as the post-market surveillance (PMS) and vigilance activities concerning those devices (MDR recital 34, IVDR recital 33). Moreover, where a manufacturer is located outside the EU and an Authorised Representative (AR) is required, a secondary control is expected to be conducted by the PRRC of the AR in order to verify regulatory compliance of the devices produced by those manufacturers.
Both the manufacturers and the Authorized Representative are required to have within their organisation, or at their disposal, at least one PRRC who possesses the proper expertise and qualification in the field of medical devices or in vitro medical devices, as applicable, in the European Union. The responsibilities of the PRRC are also set in the Regulations and interpreted in the MDCG Guidance 2019-7, which may be reviewed in due course.
The sum of required qualifications and professional experience of the PRRC are either of the following as specified in Article 15 and as clarified by the MDCG Guidance 2019-7.
Table 1: Summary of the required qualifications for the PRRC
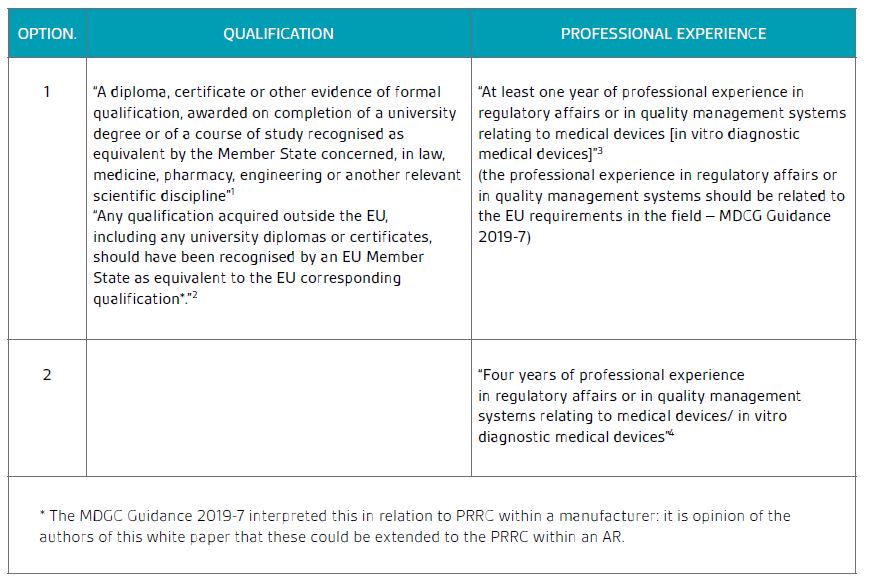
The professional experience in regulatory affairs or in quality management systems is expected to be recent in relation to EU medical devices regulation, therefore this has to be taken into consideration when appointing the PRRC.
As per MDR Article 15(1), manufacturers of custom-made devices may simply demonstrate the requisite expertise described in the first option with at least two years of professional experience within a relevant field of manufacturing.
As outlined in Table 1, the MDCG Guidance 2019-7 states that any qualification acquired outside the EU, including any university diplomas or certificates, should be recognised by an EU Member State as equivalent to the EU corresponding qualification.
Individual governments of EU countries may apply their own rules in terms of whether to recognise academic qualifications obtained elsewhere.
The website for ENIC (European Network of Information Centres in the European Region) and NARIC (National Academic Recognition Information Centres in the European Union)5 enables the user to find information
on procedures for the recognition of foreign qualifications in the different EU member states.
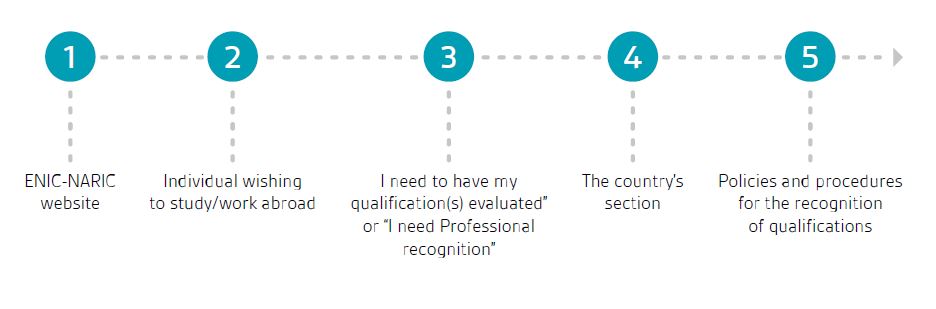
As per the ENIC-NARIC website, in order to know the details about an official national credential evaluation centre or service, the first step is to contact the national authority in charge of higher education issues in the member state where the PRRC needs an evaluation, as each country has specific rules and procedures to evaluate foreign qualifications.
Once logged into the above website, there is a box entitled “Individual wishing to study/work abroad”, where individuals will find the possibility to select “I need to have my qualification(s) evaluated” or “I need Professional recognition” pages to obtain more information or to directly select the proper member state.
Once on the country’s section of the ENIC-NARIC website, they will find a section for “Policies and procedures for the recognition of qualifications” with a link redirecting to the member state’s local webpage.
This is an excerpt from the BSI medical devices white paper, Person responsible for regulatory compliance (PRRC) - MDR/IVDR Article 15. To browse our collection of medical device white papers, please visit the Insight page on the Compliance Navigator website.
The Compliance Navigator blog is issued for information only. It does not constitute an official or agreed position of BSI Standards Ltd or of the BSI Notified Body. The views expressed are entirely those of the authors.

