Analytical performance

Analytical performance is not a new concept for IVDs, having been described in the IVDD since 1998. Many of the same analytical performance characteristics are present in both IVDD and IVDR, but IVDR seeks to expand on what nature of data should be provided. Section 9.1 of Annex I describes the analytical performance requirements, but as all scenarios of device type and intended purpose cannot be anticipated, the onus is on the manufacturer to provide a rationale for any characteristics that are not applicable to their device. This is most evident when determining analytical performance requirements for qualitative as opposed to quantitative devices. BS ISO 16142-2:2017 directs the manufacturer to international guidance documents, which are most appropriate for aiding in study design.
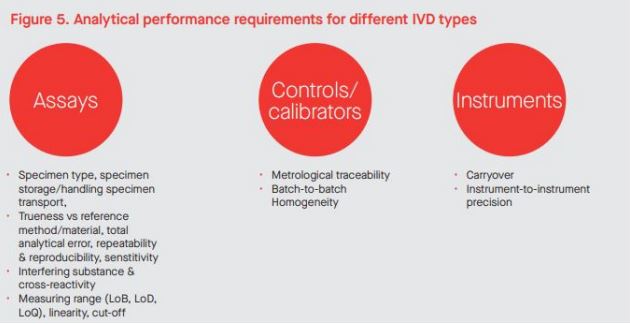
The requirements for analytical performance do not differ for Class B, C and D devices, but again, an assessment will be required on what analytical performance characteristics, if any, are appropriate for Class A devices. BS EN ISO 18113-3:2009, In vitro diagnostic medical devices — Information supplied by the manufacturer (labelling) — Part 3: In vitro diagnostic instruments for professional use, does provide some guidance on performance characteristics that should be described in the labelling for instruments. Testing for compliance with common technical specifications (to be known as common specifications once published as an implementing act for IVDR) for Class D devices can be documented under analytical performance and clinical performance, as appropriate. Figure 5 lists typical studies that would be considered analytical performance.
The IVDR does not provide guidance on the content and layout of the analytical performance report (APR), other than to state in Annex XIII 1.2.2 that ‘the manufacturer shall demonstrate the analytical performance of the device in relation to all the parameters described in point (a) of Section 9.1 of Annex I, unless any omission can be justified as not applicable. As a general rule, the analytical performance shall always be demonstrated on the basis of analytical performance studies’. Therefore as a minimum, the APR will need to link back to the PEP, describe the studies performed in sufficient detail, provide an explanation of why certain performance characteristics are not applicable and support the claims being made in the instructions for use (IFU).
This blog post is an excerpt from our latest whitepaper: Performance evaluation under IVDR. Please download the full whitepaper to find out more information.
The Compliance Navigator blog is issued for information only. It does not constitute an official or agreed position of BSI Standards Ltd or of the BSI Notified Body. The views expressed are entirely those of the authors.

